

Accredited courses by and for health professionals
 |
 Accredited courses by and for health professionals |
ONLINE EDUCATIONCOMPANY INFOWIME DIVISIONS |
Endoscope Cleaning Nursing courses are approved in all 50 states. For more accreditation information, click here.
An endoscope is, in the broadest sense, any instrument used to see inside of the body. In common use, however, the term endoscope focuses on those instruments possessing an internal light source and having the adaptability and flexibility to see into what were once hidden parts of the human body. Endoscopes are used for the diagnosis and treatment of numerous medical conditions and are part of a family of valuable diagnostic and therapeutic tools. Unfortunately, endoscopes have been traced to more healthcare-associated disease outbreaks than any other medical device. This is partly due to the nature of the tool. Flexible endoscopes, especially, by virtue of the types of body cavities they are used to visualize, acquire high levels of microbial contamination (bioburden) during use. In order to prevent the spread of nosocomial (treatment-induced) infections, all heat-sensitive endoscopes (eg, gastrointestinal endoscopes, bronchoscopes, nasopharyngoscopes—which cannot be sterilized) must be properly cleaned and subjected to high-level disinfection following each use. This course covers the critical cleaning and disinfection of these delicate and essential tools. 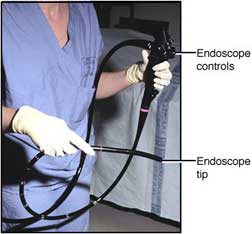
Figure 1 An endoscope. Courtesy of AstraZeneca and the Fred Hutchinson Cancer Research Center, University of Washington, Seattle. HISTORYThe concept of endoscopes has been around for a long of time. The earliest recorded references to endoscopy date back to ancient times. Hippocrates described the use of a tool to examine the rectum that is similar to a speculum. As far back as 1585 the first endoscopic procedure using a light source was achieved; it utilized sunlight focused through a flask of water to project the light into the nasal cavity (Mishra, 2002). Over the past century the science and art of endoscopy has grown more sophisticated. Instrumentation is more intricate, and at times even delicate. What continues to be true today, as it was in the time of Hippocrates, is that the high-tech tools used for invasive procedures must be maintained with great care—endoscopes, as infection control statistics and multimillion-dollar lawsuits have shown, most of all (Walker, 2001). OVERVIEWOur mission is the successful decontamination and cleaning of endoscopes and our goal is 100% disinfection; nothing less is acceptable. The tools at our disposal for disinfection and cleaning are just as high-tech as the instruments themselves, and it is essential to use the proper tool for the job. A rule that will be repeated throughout this course is “Follow the manufacturer’s instructions.” Parts and components in two very similar instruments may be made from different composite materials and the process recommended for cleaning one endoscope might damage another. Nonmanufacturer replacement parts may differ in some seemingly insignificant way so, if it doesn’t look quite right, take the time to check it out. Manufacturer cleaning instruction sheets should be placed in the work area next to the MSDS (materials safety and data sheets) and the facility’s procedure manual. Any conflicts between the procedure manual and manufacturer cleaning guidelines should be brought to the attention of the appropriate manager for resolution with the manufacturer representative. Not only might the instrument be cleaned in an improper and ineffective manner but also the warranty on a costly piece of equipment may be voided. PHYSICAL PLANTUniversal Precautions, which were adopted by the healthcare industry in 1988, emphasize the need to consider all body fluids as potential sources of bloodborne pathogens. Together with Standard Precautions, which were developed by the Occupational Safety and Health Administration (OSHA) to mandate protection for all employees who anticipate contact with blood and other potentially infectious body fluids, the concept of Universal Precautions helps structure the physical environment in which endoscope cleaning occurs. (It is becoming commonplace to use Standard Precautions to refer to the combined precautions.) In the optimal situation, the area where instruments are decontaminated is located in a room separate from the area where clean items are handled and from patient care areas. This workroom should have good ventilation and, if possible, negative pressure, with air flowing into the room rather than out. Room surfaces should be made of materials that can withstand frequent washing and wet vacuuming. Adequate workspace is provided to accommodate simultaneous washing, soaking, and rinsing of instruments. The flow of traffic in and out of the room should be restricted to prevent airborne transmission of microorganisms and transmission of contaminants that are present on the instruments. 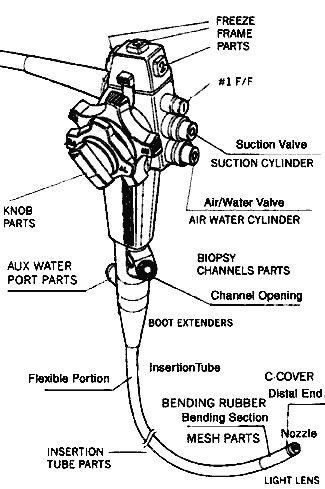
Figure 2 Note the many features of the endoscope. Endoscopes vary in size depending on their purpose. PERSONAL PROTECTIVE EQUIPMENTEach person working with contaminated instruments needs to be familiar with infection control principles and use common sense when handling infectious material. This is for personal protection, for the safety of coworkers, and for the health of the end user of the equipment they are cleaning. The person handling the instruments should wear an impervious cover gown or apron over a scrub suit or uniform. This will prevent direct contact with contaminated items and work surfaces. Hair should be covered with a disposable cap or a reusable head covering that is washed daily. Protective eyewear with side shields and mask should be worn to protect the eyes, nose, and mouth from splashes and aerosol contact. Face shields that cover the nose and mouth may be used. Heavy-duty gloves with long cuffs should be utilized to protect the hands and forearms. The use of hypoallergenic, powderless, or latex-free gloves may decrease the chance of sensitization. Sturdy shoes with nonskid soles are worn to provide support and decrease the incidence of slips and falls because it is realistic to expect the presence of intermittent surface moisture in this environment. PROCEDURE FOR CLEANINGPrecleaning and Block TestEndoscopes must be inspected frequently: before use, during use, immediately after decontamination, during the leak- and block-testing process, and prior to disinfection. Precleaning, or the removal of visible contaminants, is the single most important step in the processing of flexible endoscopes. Up to 99.8% of the bioburden can be removed in precleaning, which is thus an essential part of the process leading to the final goal of 100% disinfection. The steps for precleaning endoscopes come together as a choreographed dance routine. The first step is to don the appropriate personal protective equipment (PPE). Begin the precleaning process the moment the endoscope is removed from the patient by wiping the insertion tube with a premixed enzymatic detergent solution. As soon as possible, while still in the procedure area, suction water and/or an enzymatic detergent through all channels of the instrument, alternating air and water through the air/water channel. Suctioning detergent through all endoscope channels, alternating with air, continues until the solution appears clean.
If a channel is blocked and the enzymatic detergent and water will not flow freely through it, use a channel brush to irrigate the channel until the blockage is removed. Once removed from the light source and fluid container, be sure the protective video cap is secured and place the endoscope in a leakproof container for immediate return to the cleaning area. As in any procedure, including the cleaning of the air and water channels, remember to follow the manufacturer's instructions.
Leak TestingLeak testing determines whether the instrument is functioning properly. Initiate the leak test by attaching water-resistant caps to the endoscope electrical connections. Next, perform a visual inspection of the endoscope for holes, tears, or damage to the insertion tube. Not surprisingly, most leaks are difficult to spot with the naked eye, so attach a leakage tester and pressurize. Inspect the flexible part of the tube to ensure that the endoscope is pressurized, submerge the endoscope tip in water, and check for a stream of bubbles. While the instrument is submerged in water, turn it in all directions and continue to watch for air leaks, particularly in the bent area of the tubing. Once this is completed, curl up the insertion tube and light carrier and submerge the entire endoscope in water. Make sure the holding basin is large enough that the instrument will not have to be coiled too tightly, and watch carefully for leaks in the control knob, insertion tube, channel distal tip, valve ports, and connecters.
If the endoscope is leaking, follow the manufacturer's instructions for proper disinfection, carefully label the instrument and pack it to be sent to an authorized repair facility. Manual CleaningOnce the leak test has been completed, intact endoscopes may be cleaned and disinfected manually. Begin by preparing a detergent solution according to the manufacturer's recommendations. Remember that detergents containing surfactant are difficult to rinse from endoscope channels, and may cause blocked channels. Special detergents such as ENZOL® enzymatic detergent are available and are designed to penetrate and break down protein and organic matter. Check the manufacturer cleaning instructions to see which product is best for your specific equipment. Certain brands of detergent are temperature-dependent—ie, they need to be at a specific temperature for the enzymatic reaction to work correctly—so be aware of the particular needs of the materials you are using. Remember that the cost of a good endoscope may be considerable, so use the product that will give the greatest longevity to your scopes. The next step is to detach all removable parts, soak and/or scrub them with an endoscope cleaning brush to remove visible bioburden then rinse with water. Immerse the endoscope in solution and clean the exterior thoroughly with a brush, including the areas around the channel openings. Working from the distal end of the endoscope, use the channel cleaning brush to clean the biopsy and suction channels. Clean the brush each time it emerges from the channel. Repeat the brushing until the brush is visibly clean when it emerges from each channel. Clean the inside of the valves and biopsy port with a brush or applicator. Attach cleaning adapters to the endoscope and soak in a detergent solution for the recommended time, typically 1 to 5 minutes depending on the manufacturer. Now rinse the endoscope and all of the removable parts thoroughly with water to remove the detergent. Flush the channels with water for 60 seconds each, and use suction to purge them with air for 60 seconds. Dry the endoscope exterior and valves, using a soft-tipped applicator if needed (FPN, 2002).
DisinfectionDisinfection is designed to kill any residual biologic agents that might have escaped the physical process of cleaning. Spores, viruses, and bacteria are incredibly resilient organisms. For disinfecting, use a high-level disinfectant (HLD), making sure you follow the manufacturer’s guidelines and your facility’s MSDS. Test for minimum effective concentration with the appropriate test strips. Immerse the endoscope and valves in a basin of HLD and fill all endoscope channels with the solution. Cover the basin with a lid, following your facility’s MSDS procedures for the containment of any irritating vapors that might be released. Soak the endoscope long enough to achieve high-level disinfection, based on the manufacturer’s guidelines, the MSDS and your facility’s procedure manual. After the allotted time, remove the endoscope from the basin and thoroughly rinse the exterior and the channels in a basin of sterile water. Nonsterile water may be used if indicated by agency policy. Perform the rinsing process three times, using a fresh supply of water for each rinse. This ensures that any residual disinfectant has been safely removed. Dry the exterior of the endoscope with a soft lint-free cloth and remove any adapters that have been applied. Purge all of the channels with air and flush with 70% alcohol if indicated by the manufacturer guidelines and by agency policy. Flush with alcohol and purge the channels with air to remove the alcohol. This has been found to greatly reduce the possibility of recontamination of the endoscope by waterborne microorganisms in both manual and automated systems (FDA, 1999).
At this point you may remove your protective attire, not forgetting to wash your hands in accordance with Standard Precautions. Hang the endoscope vertically in a clean, dry storage cabinet to prevent air particulate and dust contamination. Keep the valves separate in order to promote adequate airflow and avoid the risk of condensation. Automatic Cleaning and DisinfectionAutomation tends to bring both blessings and curses, and so it is with endoscope automatic reprocessors. As with the manual cleaning method, once the leak and block tests have been successfully completed, the intact endoscope may be cleaned and disinfected using an automatic reprocessor. Remember to follow the manufacturer's instructions for the specific brand of automated reprocessor your agency is using. Begin by placing the endoscope in the reprocessor basin and connect the adapters to all channels and ports. The valves and other removable parts should be placed in the designated area of the reprocessor. It is acceptable at this time to remove protective attire and wash your hands, as the remainder of the process takes place in a contained environment. Once the reprocessor lid is closed, set the parameters of the machine for the processing time required by the enzymatic cleaning agent and the high-level disinfectant (HDL) that you are using. Information on dilutions and times can be found in the MSDS and in the use instructions that come with the reprocessor. When the reprocessor cycle is complete, disconnect all of the adapters and remove the endoscope from the basin. Dry the exterior of the unit with a soft lint-free cloth and flush the channels with 70% alcohol prior to storage, as indicated by agency policy or manufacturer instructions. When the alcohol flush is completed purge the channels with air to remove any alcohol residue. Studies have found that this step, which utilizes the denaturing and evaporative characteristics of alcohol, can greatly reduce the possibility of recontamination of the endoscope by waterborne microorganisms. Endstage recontamination has been tracked back to the use of reprocessors in some cases of serious hospital-induced infections. An additional few minutes of time is well worth the safety that this final step provides (FDA, 1999). Again hang the endoscope vertically for storage in a clean, dry cabinet with valves removed to promote adequate airflow and prevent condensation. CONCLUSIONA patient should not have to fear infection from an improperly cleaned endoscope when coming to the hospital for a potentially lifesaving procedure. With proper education and good technique it is within our control to completely eliminate endoscope-related infections. The goal is 100% disinfection with absolutely no risk of infection to those who depend on us for good medical care. We can do it by using proper procedures and techniques. This goal is entirely obtainable. We will do it. Lives depend on us. Posted April 1, 2003 Expires October 1, 2005 Copyright © 2003 Wild Iris Medical Education. All rights reserved. REFERENCESFood and Drug Administration (FDA). (1999). Infections from Endoscopes Inadequately Reprocessed by an Automated Endoscope Reprocessing System. FDA and CDC Public Health Advisory. Retrieved in September 1999 from http://www.fda.gov/cdrh/safety/endoreprocess.html. Mishra R. (2002). History of Minimal Access Surgery. Retrieved in June 2002 from http://www.laparoscopyhospital.com. Rutala W, et al. (2002). Draft Guidelines for Disinfection and Sterilization in Healthcare Facilities. Healthcare Infection Control Practices. Retrieved in June 2002 from the CDC website. Walker A. (2001). Hospitals guilty of poor endoscope cleaning? Medical Post, May 2001. Family Practice Notebook (FPN). (2002). Endoscope Cleaning. Retrieved from http://www.fpnotebook.com. | ||||||||||||||||||||||||
 Accreditation
Home Accreditation
HomeSite Map Contact Privacy Disclaimer NursingCEU.com is a division of Wild Iris Medical Education Copyright © 2005 Wild Iris Medical Education | |||||||||||||||||||||||||